Introduction: Acute Myeloid Leukemia (AML) is marked by uncontrolled growth of undifferentiated myeloid cells. DNA methylation patterns in AML have been thoroughly researched, however, studies on hydroxy methylation patterns, indicating active DNA methylation erasure, are still evolving. Our principal goal was to detect differential methylation and hydroxy methylation regions between AML and non-malignant samples. We proposed that simultaneous profiling of blood cells and circulating cell-free DNA would offer more insights into the disease mechanism. Therefore, we combined these data sources to provide a comprehensive epigenetic picture, assuming that this dual approach could reveal new AML biomarkers and enhance our understanding regarding AML pathobiology.
Methods: We collected peripheral blood samples from 16 newly diagnosed AML patients (median age 64, age range 36-85) treated at Tel Aviv Sourasky Medical Center, Israel, and 33 age-matched healthy volunteers (NCT05735704). We utilized novel microarray technology to examine methylation and hydroxy methylation patterns in both genomic and cell-free DNA. Following differential methylation region extraction, we used DAVID functional annotation tool to interpret our results' biological significance.
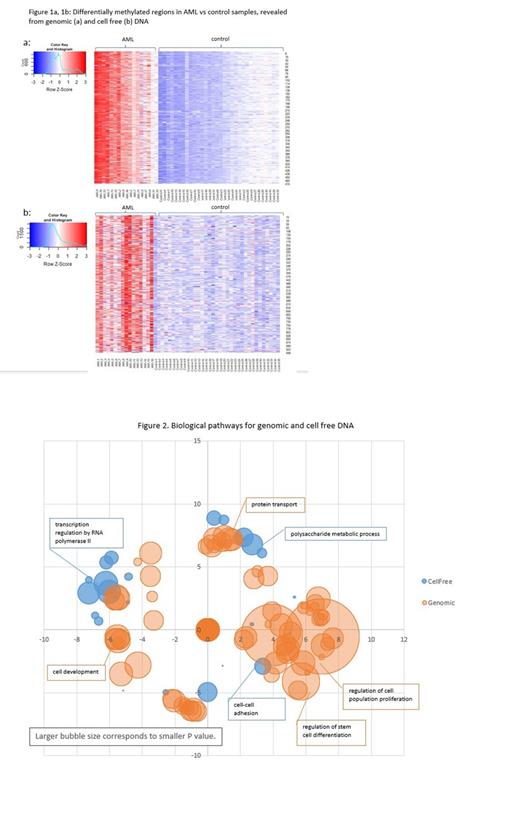
Results: This report presents the preliminary methylation data results. Methylation analysis unveiled 474 and 475 differentially methylated regions between AML and controls in genomic and cell-free DNA analysis, respectively, with only 18 common regions. The clear AML-control distinction is displayed in hierarchical clustering heatmaps (Figure 1a, 1b).
Further DAVID tool analysis revealed enrichment in biological processes such as aging, cell cycle regulation, cell morphogenesis, and MAP kinase activity - processes closely linked with AML. Notably, genomic data analysis showed significant enrichment of the ‘transcriptional misregulation in cancer’ pathway, involving genes encoding vital transcription factors for cell development and differentiation. Figure 2 demonstrates the biological pathways for genomic and cell free data analysis.
Conclusions: This research underscores the importance of combined epigenetic data from malignant tissue and its microenvironment, obtained by examining genomic and cell-free DNA. Both datasets exhibit striking differences between AML and control samples. We detected enrichment of AML-related biological pathways within differential regions. Our findings of enriched biological pathways align with existing literature, reinforce our analysis validity, and support our combined view of methylation patterns in genomic and cell-free DNA. By suggesting a combined view of these two types of DNA, we provide a more comprehensive perspective on AML's complex epigenetic landscape. It underscores the value of such integrative epigenetic profiling in shedding new light on the intricacies of AML development and progression, which may eventually guide the discovery of novel therapeutic targets.
Disclosures
Avivi Mazza:AbbVie: Honoraria. Moshe:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Stemline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.